Research
Research synopsis
Proteins and small molecules are translocated across lipid bilayers by integral membrane proteins that span the bilayer and facilitate translocation. My lab centers around determining the atomic resolution structure of membrane proteins and complexes that function to transport materials across lipid bilayers. The primary focus of research in the lab focuses on nutrient uptake mechanisms within pathogenic Gram negative bacterial species . In addition to the structural and biochemical characterization of these ion transport system components, research in my lab examines the membrane protein complexes that facilitate the proper insertion and assembly of these membrane protein transport components including surface anchored lipoproteins.
Slam-dependent surface lipoprotein translocation
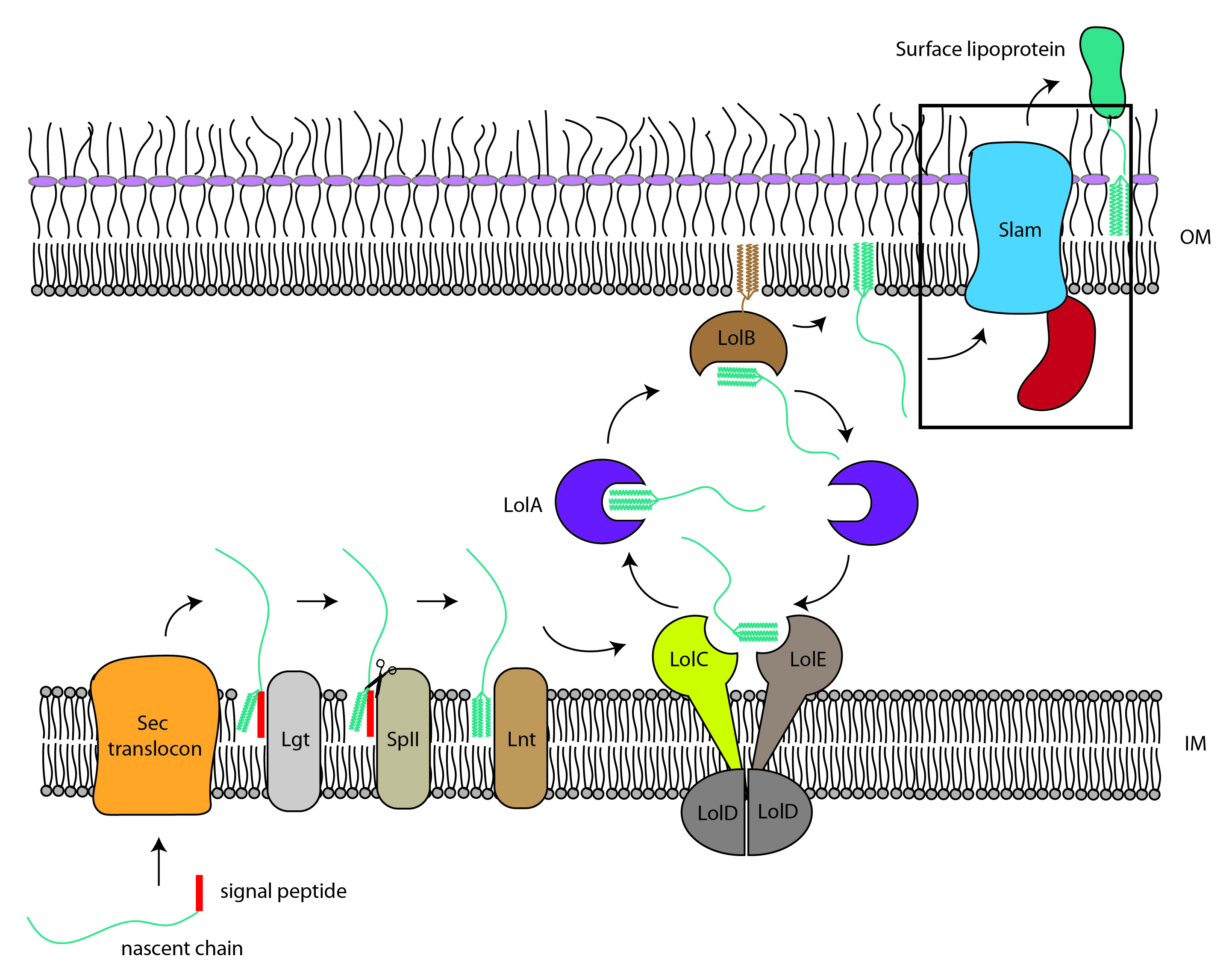
Lipoproteins decorate the surface of many obligate host restricted Gram-negative bacterial pathogens, playing essential roles in immune evasion and nutrient acquisition. In Neisseria spp., the causative agents of gonorrhea and meningococcal meningitis, surface lipoproteins (SLPs) such as factor H-binding protein (fHbp) and transferrin binding protein B (TbpB) are required for virulence and are primary targets for broad-spectrum vaccine development since they elicit bactericidal antibodies. The surface lipoprotein assembly modulator (Slam) is required for the proper assembly of SLPs on the surface of Gram negative bacteria and is required for virulence. The mechanism of Slam and the translocation of SLPs is fundamental for the survival of these host restricted bacteria.
Figure 1: Slam-dependent SLP translocation
Engineering SLPs for Use in Vaccines
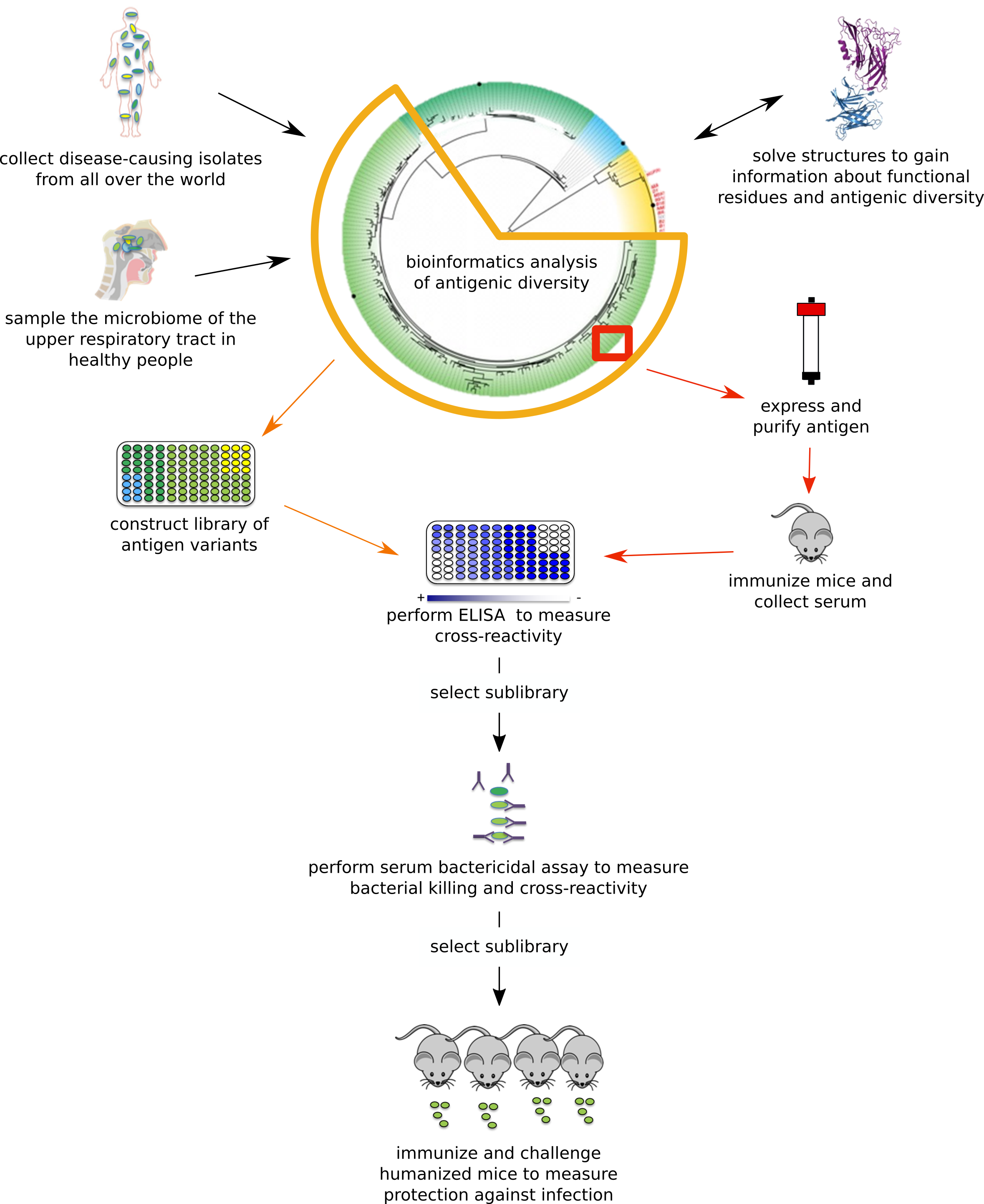
SLPs are located on the bacterial surface and involved in processes key to bacterial survival and virulence. We engineer SLPs to produce antigens that have the potential to be more protective against bacterial infections than their corresponding wild-type variants. Together with collaborators, we have constructed a vaccine development pipeline (Figure 2). Because our goal is to produce vaccines that are broadly protective, we select SLP orthologs from diverse bacterial strains, ensure that they can be expressed and purified in a soluble form, and test for their ability to elicit production of cross-reactive antibodies that and protect against infection in mice.
Figure 2: Vaccine development pipeline
Phosphorylated-Carbohydrate Uptake
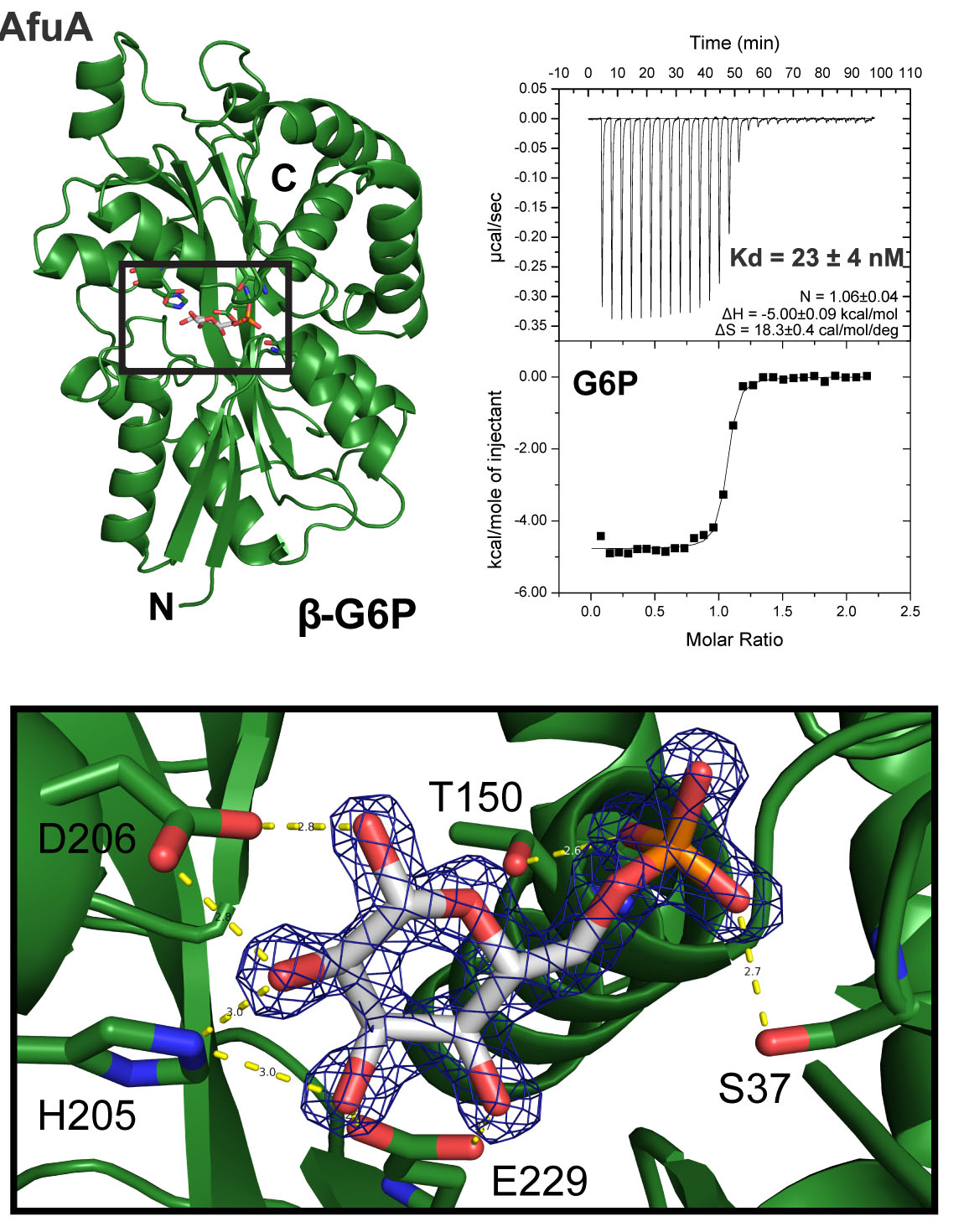
Disease-causing bacteria depend on the acquisition of a diverse set of nutrients from their hosts to engage in successful pathogenesis. AfuABC binds and transports a particular set of phosphorylated carbohydrates (glucose-6-phosphate, fructose-6-phosphate and sedoheptulose-7-phosphate). AfuABC is conserved across bacteria, including a large number of Gram-negative pathogens such as Vibrio cholerae, Haemophilus influenzae and Citrobacter rodentium. AfuABC can mediate sugar-phosphate transport into bacteria and C. rodentium lacking AfuA are significantly impaired in competitive growth within the mammalian gut indicating that AfuABC based recognition and uptake of sugar-phosphates within the intestinal lumen plays a critical role during infection.
Figure 3: The Periplasmic Glucose-6-phosphate binding protein, AfuA
Iron acquisition through the bacterial transferrin receptor
In the vertebrate host, the level of free extracellular iron is well below that required to support the growth of bacterial pathogens, largely owing to the iron-sequestering effects of iron-binding glycoproteins transferrin and lactoferrin. Successful bacterial pathogens have developed high-affinity iron uptake systems capable of acquiring iron from transferrin and lactoferrin. Members of the Neisseriacea and Pasteurellaceae family including Neisseria meningitidis and Haemophilus influenza possess receptors consisting of the surface exposed lipoprotein TbpB and the integral outer membrane protein TbpA that bind transferrin and are involved in the retrieval and transport of iron across the outer membrane. Within the periplasm, the ferric binding protein, FbpA, binds iron and escorts it to the inner membrane ABC transporter where it is transported into the cytoplasm (Figure 4).
Figure 4: Iron Acquisition via the Bacterial Transferrin Receptor

The structural information gained about the conserved interactions between these essential outer membrane proteins and transferrin will represent the target regions for the development of broad-spectrum vaccines against these highly infectious bacterial pathogens.
The SLC26 Anion Transporter, YchM
The human solute carrier SLC26 family of anion transporters consists of 11 members that are expressed in polarized cells in organs such as the kidney, pancreas, intestine and liver where they mediate sulfate, chloride and bicarbonate transport across the plasma membrane. The SLC26 transporter family is widely-expressed and includes SulP transporters that are commonly found in bacteria, fungi and plants where they mediate sulfate or bicarbonate transport. In collaboration with the Reithmeier Lab we will investigate the structural and biochemical activities of the YchM transporter from E. coli, which is homologous to SLC26 family of bicarbonate transporters within humans. Recently we determined the high-resolution crystal structure of the STAS domain from E. coli YchM isolated in complex with acyl-carrier protein (ACP), an essential component of the fatty acid biosynthesis (FAB) pathway (Figure 3).
Figure 5: The STAS domain of YchM functions in Fatty Acid Metabolism

We concluded that YchM, a bacterial member of the SLC26 family of anion transporters, is linked to fatty acid metabolism via a direct interaction with ACP. The major goal of our work will be to define structure of the membrane protein YchM in order to illuminate the mechanism of bicarbonate transport and its role in fatty acid metabolism.